Isomerism in Coordination Compounds
Isomerism in Coordination Compounds: Overview
This Topic covers sub-topics such as Optical Isomerism, Geometrical Isomerism, Stereoisomerism, Structural Isomerism, Isomerism in Coordination Compounds, Linkage Isomerism, Coordination Isomerism, Optically Active Compounds and, Ionization Isomerism
Important Questions on Isomerism in Coordination Compounds
In 20th century, German scientist Werner succeeded in clarifying the structures of the five compounds consisting of platinum, chlorine, and ammonia. Some of the properties of these compounds are shown below in the table.
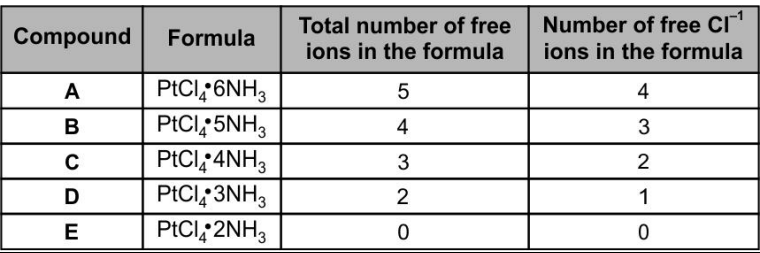
Which of the complexes formed for the compounds have structural isomers?
The complexhas two isomers whereas does not show geometrical isomerism and has no isomers why?
Optical isomerism is expected in tetrahedral complexes of the type analogous to tetrahedral carbon atom.
Complex can not show optical isomerism.
Write the geometrical isomers of .
Draw the structure of trans isomers of diamminedichloroplatinum(II).
Draw the structure of cis isomers of diamminedichloroplatinum(II).
Geometrical isomerism is possible in?
Why is geometrical isomerism not possible in tetrahedral complexes having two different types of undentate ligands coordinated with the central metal ion?
Identify the isomer occur in the given ligand. _____ (Facial (fac) isomer/ Meridional (mer) isomer)
Define Facial (fac) isomer.
If three donor atoms of the same ligands occupy adjacent positions at the corners of an octahedral face, we have the facial (fac) isomer.
State the method to draw the different geometrical forms for a particular complex in octahedral geometry.
State the method to draw the different geometrical forms for a particular complex in square planer geometry.
_____ planar complexes do not show optical isomerism.
Square planar complexes show optical isomerism.
What is coordination isomerism in coordination compounds ?
What is Structural isomerism How is it classified?
Due to the presence of ambidentate ligands coordination compounds show isomerism. Palladium complexes of the type and are _____(linkage isomers/ionisation isomers)
Draw cis and trans geometrical isomers of .
